Preparation and Evaluation of Orodispersible Films Containing Spray-Dried Rifampicin Nanosuspension
Abstract
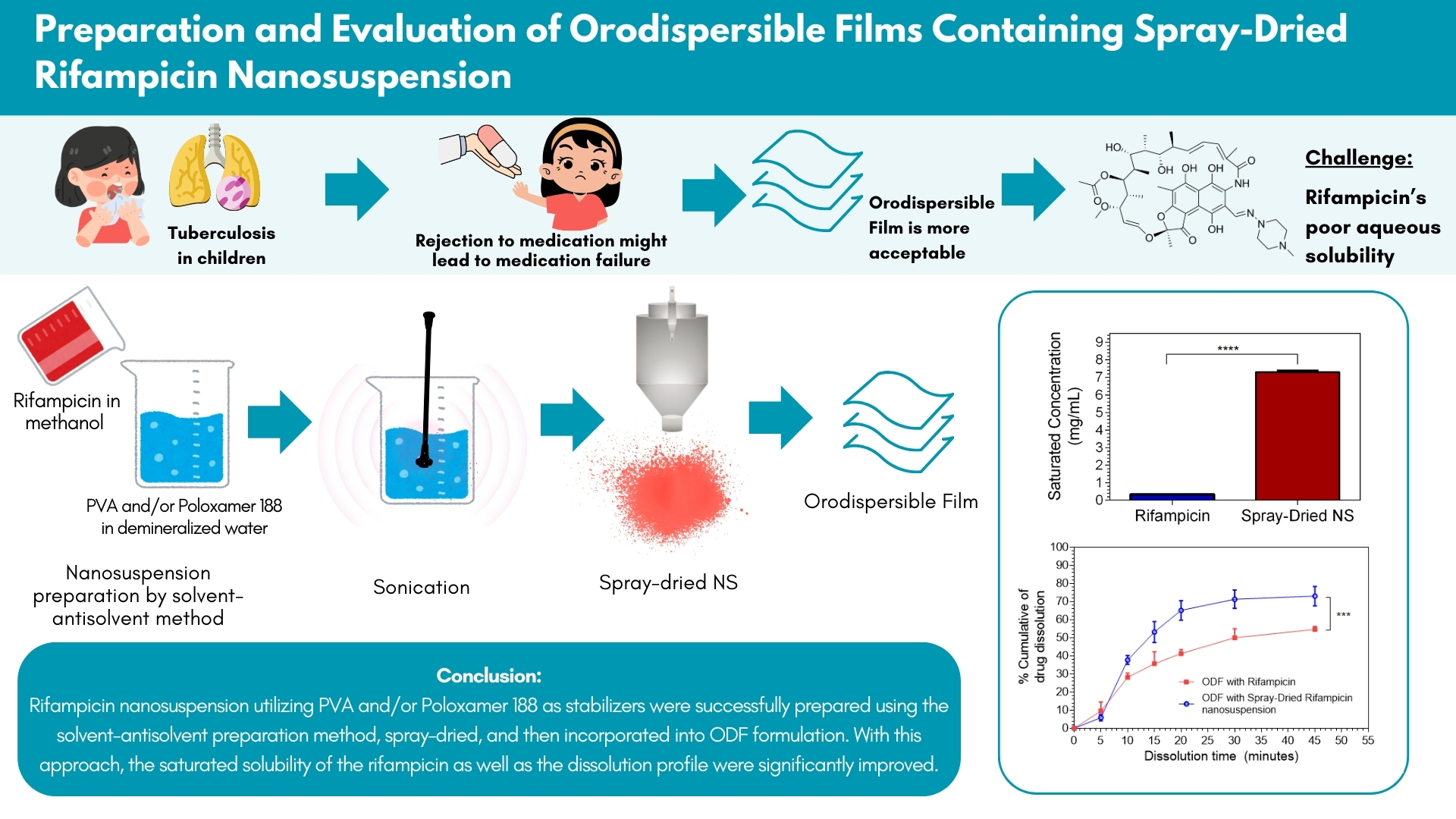
Background: Tuberculosis remains a major global health challenge, with high mortality rates. Conventional anti-TB dosage forms like tablets are often unsuitable for patients who struggle with swallowing, leading to poor compliance. To overcome these limitations, this research aimed to develop an orodispersible film (ODF) containing rifampicin, a widely used anti-tuberculosis drug. However, rifampicin’s low aqueous solubility poses challenges for its incorporation into ODFs and affects its therapeutic effectiveness. To address these issues, rifampicin was first prepared as a nanosuspension and subsequently dried before being formulated into the ODF.
Methods: Nanosuspensions were stabilized using polyvinyl alcohol (PVA), poloxamer 188 (POX), and their combination at various concentrations. The nanosuspensions were prepared using solvent-antisolvent precipitation followed by sonication and spray-drying. The resulting spray-dried nanosuspension was characterized, and the optimal formula was used in ODF formulation through the solvent-casting method.
Results: Formulations PV2 (PVA 0.4%) and POX1 (POX 0.2%) demonstrated the smallest particle sizes at 306±14.01 nm and 291±7.55 nm, respectively. After reconstitution, PV2 maintained the particle size comparably to POX1. The spray-dried PV2 nanosuspension exhibited a 21.48-fold increase in saturated solubility compared to the pure rifampicin and superior drug release (79% vs 58%) compared to the standard rifampicin suspension. ODF containing PV2 showed improved organoleptic properties and enhanced drug dissolution (82% vs 56%) compared to original rifampicin ODF.
Conclusion: Formulating rifampicin into a nanosuspension stabilized by PVA and POX, followed by spray-drying, significantly improves its solubility and drug release profile. This approach also enhances the organoleptic properties and dissolution of rifampicin in ODF, offering a promising strategy to boost rifampicin’s therapeutic efficacy in tuberculosis treatment, particularly for pediatric patients.
Graphical Abstract:
References
Adeleke, O. A., Monama, N. O., Tsai, P.-C., Sithole, H. M., & Michniak-Kohn, B. B. (2016). Combined Atomistic Molecular Calculations and Experimental Investigations for the Architecture, Screening, Optimization, and Characterization of Pyrazinamide Containing Oral Film Formulations for Tuberculosis Management. Molecular Pharmaceutics, 13(2), 456–471. https://doi.org/10.1021/acs.molpharmaceut.5b00698
Adeleke, O. A., Tsai, P.-C., Karry, K. M., Monama, N. O., & Michniak-Kohn, B. B. (2018). Isoniazid-loaded orodispersible strips: Methodical design, optimization and in vitro-in silico characterization. International Journal of Pharmaceutics, 547(1–2), 347–359. https://doi.org/10.1016/j.ijpharm.2018.06.004
Albanna, A. S., Smith, B. M., Cowan, D., & Menzies, D. (2013). Fixed-dose combination antituberculosis therapy: a systematic review and meta-analysis. European Respiratory Journal, 42(3), 721–732. https://doi.org/10.1183/09031936.00180612
Alshweiat, A., Katona, G., Csóka, I., & Ambrus, R. (2018). Design and characterization of loratadine nanosuspension prepared by ultrasonic-assisted precipitation. European Journal of Pharmaceutical Sciences, 122, 94–104. https://doi.org/10.1016/j.ejps.2018.06.010
Chaubal, M. V., & Popescu, C. (2008). Conversion of Nanosuspensions into Dry Powders by Spray Drying: A Case Study. Pharmaceutical Research, 25(10), 2302–2308. https://doi.org/10.1007/s11095-008-9625-0
Gaaz, T., Sulong, A., Akhtar, M., Kadhum, A., Mohamad, A., & Al-Amiery, A. (2015). Properties and Applications of Polyvinyl Alcohol, Halloysite Nanotubes and Their Nanocomposites. Molecules, 20(12), 22833–22847. https://doi.org/10.3390/molecules201219884
Guan, W., Ma, Y., Ding, S., Liu, Y., Song, Z., Liu, X., Tang, L., & Wang, Y. (2022). The technology for improving stability of nanosuspensions in drug delivery. Journal of Nanoparticle Research, 24(14). https://doi.org/10.1007/s11051-022-05403-9
Holder, C. F., & Schaak, R. E. (2019). Tutorial on Powder X-ray Diffraction for Characterizing Nanoscale Materials. ACS Nano, 13(7), 7359–7365. https://doi.org/10.1021/acsnano.9b05157
Kanabalan, R. D., Lee, L. J., Lee, T. Y., Chong, P. P., Hassan, L., Ismail, R., & Chin, V. K. (2021). Human tuberculosis and Mycobacterium tuberculosis complex: A review on genetic diversity, pathogenesis and omics approaches in host biomarkers discovery. Microbiological Research, 246, 126674. https://doi.org/10.1016/j.micres.2020.126674
Karakucuk, A., Canpinar, H., & Celebi, N. (2022). Ritonavir nanosuspensions prepared by microfluidization with enhanced solubility and desirable immunological properties. Pharmaceutical Development and Technology, 27(10), 1027–1037. https://doi.org/10.1080/10837450.2022.2145309
Li, J., Wang, Z., Zhang, H., Gao, J., & Zheng, A. (2021). Progress in the development of stabilization strategies for nanocrystal preparations. Drug Delivery, 28(1), 19–36. https://doi.org/10.1080/10717544.2020.1856224
Liu, H., He, Z.-Z., Yu, L., Ma, J., & Jin, X.-P. (2021). Improved solubility and stability of rifampicin as an inclusion complex of acyclic cucurbit[n]uril Abbreviations RIF Rifampicin ACB Acyclic Cucurbit[n]uril CB[n] Cucurbit[n]uril DSC Differential scanning calorimetry. Journal of Inclusion Phenomena and Macrocyclic Chemistry, 101, 111–120. https://doi.org/10.1007/s10847-021-01093-3
Mehanna, M. M., Mohyeldin, S. M., & Elgindy, N. A. (2019). Rifampicin-Carbohydrate Spray-Dried Nanocomposite: A Futuristic Multiparticulate Platform For Pulmonary Delivery. Int J Nanomedicine, 14, 9089–9112. https://doi.org/10.2147/IJN.S211182
Preis, M., Knop, K., & Breitkreutz, J. (2014). Mechanical strength test for orodispersible and buccal films. International Journal of Pharmaceutics, 461(1–2), 22–29. https://doi.org/10.1016/j.ijpharm.2013.11.033
Ruecroft, G., Hipkiss, D., Ly, T., Maxted, N., & Cains, P. W. (2005). Sonocrystallization: The Use of Ultrasound for Improved Industrial Crystallization. Organic Process Research & Development, 9(6), 923–932. https://doi.org/10.1021/op050109x
Santoveña-Estévez, A., Suárez-González, J., Cáceres-Pérez, A. R., Ruiz-Noda, Z., Machado-Rodríguez, S., Echezarreta, M., Soriano, M., & Fariña, J. B. (2020). Stability Study of Isoniazid and Rifampicin Oral Solutions Using Hydroxypropyl-Β-Cyclodextrin to Treat Tuberculosis in Paediatrics. Pharmaceutics, 12(2), 195. https://doi.org/10.3390/pharmaceutics12020195
Son, Y., No, Y., & Kim, J. (2020). Geometric and operational optimization of 20-kHz probe-type sonoreactor for enhancing sonochemical activity. Ultrasonics Sonochemistry, 65, 105065. https://doi.org/10.1016/j.ultsonch.2020.105065
Sun, W., Ni, R., Zhang, X., Chiu Li, L., & Mao, S. (2015). Spray drying of a poorly water-soluble drug nanosuspension for tablet preparation: formulation and process optimization with bioavailability evaluation Spray drying of a poorly water-soluble drug nanosuspension for tablet preparation: formulation and proc. Drug Dev Ind Pharm, 41(6), 927–933. https://doi.org/10.3109/03639045.2014.914528
Sutradhar, K. B., Khatun, S., & Luna, I. P. (2013). Increasing Possibilities of Nanosuspension. Journal of Nanotechnology, 346581, 12. https://doi.org/10.1155/2013/346581
Tehrani, A. A., Omranpoor, M. M., Vatanara, A., Seyedabadi, M., & Ramezani, V. (2019). Formation of nanosuspensions in bottom-up approach: theories and optimization. DARU Journal of Pharmaceutical Sciences, 27(1), 451–473. https://doi.org/10.1007/s40199-018-00235-2
Tewes, F., Brillault, J., Couet, W., & Olivier, J. C. (2008). Formulation of rifampicin–cyclodextrin complexes for lung nebulization. Journal of Controlled Release, 129(2), 93–99. https://doi.org/10.1016/J.JCONREL.2008.04.007
Wang, Y., Zheng, Y., Zhang, L., Wang, Q., & Zhang, D. (2013). Stability of nanosuspensions in drug delivery. Journal of Controlled Release, 172(3), 1126–1141. https://doi.org/10.1016/J.JCONREL.2013.08.006
Wei, Q., Keck, C. M., & Müller, R. H. (2018). Solidification of hesperidin nanosuspension by spray drying optimized by design of experiment (DoE). Drug Development and Industrial Pharmacy, 44(1), 1–12. https://doi.org/10.1080/03639045.2017.1285309
Wu, L., Zhang, J., & Watanabe, W. (2011). Physical and chemical stability of drug nanoparticles. Advanced Drug Delivery Reviews, 63(6), 456–469. https://doi.org/10.1016/J.ADDR.2011.02.001
Xia, D., Shrestha, N., van de Streek, J., Mu, H., & Yang, M. (2016). Spray drying of fenofibrate loaded nanostructured lipid carriers. Asian Journal of Pharmaceutical Sciences, 11(4), 507–515. https://doi.org/10.1016/j.ajps.2016.01.001







