Spatial learning and memory of young and aging rats following injection with human Wharton’s jelly‐mesenchymal stem cells
Berry Juliandi(1*), Wildan Mubarok(2), Dian Anggraini(3), Arief Boediono(4), Mawar Subangkit(5), Indra Bachtiar(6), Harry Murti(7), Kelvin Yaprianto(8), Boenjamin Setiawan(9)
(1) Department of Biology, Faculty of Mathematics and Natural Sciences, Bogor Agricultural University (IPB University), Kampus IPB Dramaga, Bogor 16680
(2) Department of Biology, Faculty of Mathematics and Natural Sciences, Bogor Agricultural University (IPB University), Kampus IPB Dramaga, Bogor 16680
(3) Department of Biology, Faculty of Mathematics and Natural Sciences, Bogor Agricultural University (IPB University), Kampus IPB Dramaga, Bogor 16680
(4) Department of Anatomy, Physiology, and Pharmacology, Faculty of Veterinary Medicine, Bogor Agricultural University (IPB University), Kampus IPB Dramaga, Bogor 16680
(5) Department of Veterinary Clinic Reproduction, Faculty of Veterinary Medicine, Bogor Agricultural University (IPB University), Kampus IPB Dramaga, Bogor 16680
(6) Stem Cell Division, Stem Cell and Cancer Institute, Jl. A. Yani No. 2 Pulo Mas, Jakarta 13210
(7) Stem Cell Division, Stem Cell and Cancer Institute, Jl. A. Yani No. 2 Pulo Mas, Jakarta 13210
(8) Stem Cell Division, Stem Cell and Cancer Institute, Jl. A. Yani No. 2 Pulo Mas, Jakarta 13210
(9) Stem Cell Division, Stem Cell and Cancer Institute, Jl. A. Yani No. 2 Pulo Mas, Jakarta 13210
(*) Corresponding Author
Abstract
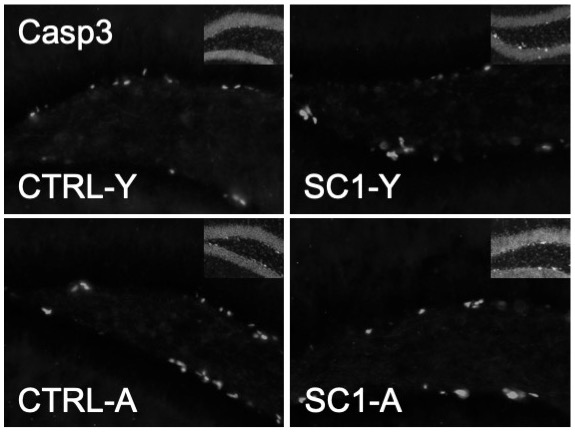
Human Wharton’s jelly‐mesenchymal stem cells (hWJ‐MSC) are an emerging potential source of stem cells derived from the umbilical cord. Previous studies have shown their potential as treatment for traumatic brain injury and Parkinson’s disease. However, no study has yet investigated the effect of hWJ‐MSC injections in countering spatial learning and memory impairment in aging rats. The effect of hWJ‐MSC injection on young rats is also unknown. The objective of this research was to analyze the effect of an hWJ‐MSC injection on spatial learning, memory, density of putative neural progenitor cells (pNPC), and neuronal apoptosis in the dentate gyrus (DG) of young and aging rats. Injection of hWJ‐MSC did not change spatial learning and memory in young rats until two months post‐injection. This might be due to retained pNPC density and neuronal apoptosis in the DG of young rats after injection of hWJ‐MSC. In contrast, injection of hWJ‐MSC promoted both spatial learning and memory in aging rats, a finding that might be attributable to the increased pNPC density and attenuated neuronal apoptosis in DG of aging rats during the two months post‐injection. Our study suggests that a single injection of hWJ‐MSC might be sufficient to promote improvement in long‐term learning and memory in aging rats.
Keywords
Full Text:
PDFReferences
Antoninus AA, Agustina D, Wijaya L, Hariyanto V, Yanti MM, Setiawan B, Bachtiar I. 2012. Wharton’s Jelly–Derived Mesenchymal Stem Cells: Isolation and Characterization. Cermin Dunia Kedokteran. 39(8):588–591.
Bali P, Lahiri D, Banik A, Nehru B, Anand A. 2016. Potential for Stem Cells Therapy in Alzheimer’s Disease: Do Neurotrophic Factors Play Critical Role? Curr Alzheimer Res. 14(2):208–220. doi:10.2174/1567205013666160314145347.
Bernal GM, Peterson DA. 2004. Neural stem cells as therapeutic agents for agerelated brain repair. Aging Cell. 3(6):345–351. doi:10.1111/j.1474 9728.2004.00132.x.
Bevins RA, Besheer J. 2006. Object recognition in rats and mice: A onetrial nonmatchingtosample learning task to study ’recognition memory’. Nat Protoc. 1(3):1306–1311. doi:10.1038/nprot.2006.205.
Boyette L, Tuan R. 2014. Adult Stem Cells and Diseases of Aging. J Clin Med. 3(1):88–134. doi:10.3390/jcm3010088.
Cheng T, Yang B, Li D, Ma S, Tian Y, Qu R, Zhang W, Zhang Y, Hu K, Guan F, Wang J. 2015. Wharton’s Jelly Transplantation Improves Neurologic Function in a Rat Model of Traumatic Brain Injury. Cell Mol Neurobiol. 35(5):641–649. doi:10.1007/s10571015 01599.
Deng W, Aimone JB, Gage FH. 2010. New neurons and new memories: How does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 11(5):339–350. doi:10.1038/nrn2822.
Drapeau E, Mayo W, Aurousseau C, Le Moal M, Piazza PV, Abrous DN. 2003. Spatial memory performances of aged rats in the water maze predict levels of hippocampal neurogenesis. Proc Natl Acad Sci USA. 100(SUPPL. 2):14385–14390. doi:10.1073/pnas.2334169100.
Dupret D, Fabre A, Döbrössy MD, Panatier A, Rodríguez JJ, Lamarque S, Lemaire V, Oliet SH, Piazza PV, Abrous DN. 2007. Spatial learning depends on both the addition and removal of new hippocampal neurons. PLoS Biol. 5(8):1683–1694. doi:10.1371/journal.pbio.0050214.
Elahy M, Jackaman C, Mamo JC, Lam V, Dhaliwal SS, Giles C, Nelson D, Takechi R. 2015. Bloodbrain barrier dysfunction developed during normal aging is associated with inflammation and loss of tight junctions but not with leukocyte recruitment. Immun Ageing. 12(1). doi:10.1186/s1297901500299.
Elmore S. 2007. Apoptosis: A Review of Programmed Cell Death. Toxicol Pathol. 35(4):495–516. doi:10.1080/01926230701320337.
Floresco SB. 2010. Spatial Learning in Animals. In: IP Stolerman, editor, Encyclopedia of Psychopharmacology. Berlin: Springer. p. 1259–1262. doi:10.1007/9783540687061_354.
Galli RL, ShukittHale B, Youdim KA, Joseph JA. 2002. Fruit polyphenolics and brain aging: Nutritional interventions targeting agerelated neuronal and behavioral deficits. Ann N Y Acad Sci. 959(1):128–132. doi:10.1111/j.17496632.2002.tb02089.x.
Goncharova V, Das S, Niles W, Schraufstatter I, Wong AK, Povaly T, Wakeman D, Miller L, Snyder EY, Khaldoyanidi SK. 2014. Homing of Neural Stem Cells From the Venous Compartment Into a Brain Infarct Does Not Involve Conventional Interactions With Vascular Endothelium. Stem Cells Transl Med. 3(2):229–240. doi:10.5966/sctm.20130052.
Han YG, Spassky N, RomagueraRos M, GarciaVerdugo JM, Aguilar A, SchneiderMaunoury S, AlvarezBuylla A. 2008. Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat Neurosci. 11(3):277–284. doi:10.1038/nn2059.
Hashem HE, Elmasry SM, Eladl MA. 2010. Dentate Gyrus in Aged Male Albino Rats ( Histological and Tau Immunohistochemical Study ). Egypt J Histol. 33(4):659–670. Hattiangady B, Shetty AK. 2008. Aging does not alter the number or phenotype of putative stem/progenitor cells in the neurogenic region of the hippocampus. Neurobiol Aging. 29(1):129–147. doi:10.1016/j.neurobiolaging.2006.09.015.
Heine VM, Maslam S, Joëls M, Lucassen PJ. 2004. Prominent decline of newborn cell proliferation, differentiation, and apoptosis in the aging dentate gyrus, in absence of an agerelated hypothalamuspituitaryadrenal axis activation. Neurobiol Aging. 25(3):361– 375. doi:10.1016/S01974580(03)000903.
Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. 2002. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. pro 99(18):11946– 11950. doi:10.1073/pnas.182296499.
Juliandi B, Tanemura K, Igarashi K, Tominaga T, Furukawa Y, Otsuka M, Moriyama N, Ikegami D, Abematsu M, Sanosaka T, et al. 2015. Reduced Adult Hippocampal Neurogenesis and Cognitive Impairments following Prenatal Treatment of the Antiepileptic Drug Valproic Acid. Stem Cell Reports 5(6):996– 1009. doi:10.1016/j.stemcr.2015.10.012.
Kempermann G. 2002. Why new neurons? Possible functions for adult hippocampal neurogenesis. J Neurosci. 22(3):635–638. doi:10.1523/jneurosci.2203 00635.2002. Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH. 2003. Early determination and longterm persistence of adultgenerated new neurons in the hippocampus of mice. Development. 130(2):391–399. doi:10.1242/dev.00203.
Kim SE, Ko IG, Kim BK, Shin MS, Cho S, Kim CJ, Kim SH, Baek SS, Lee EK, Jee YongSeok YS. 2010. Treadmill exercise prevents aginginduced failure of memory through an increase in neurogenesis and suppression of apoptosis in rat hippocampus. Exp Gerontol. 45(5):357–365. doi:10.1016/j.exger.2010.02.005.
Koh SH, Kim KS, Choi MR, Jung KH, Park KS, Chai YG, Roh W, Hwang SJ, Ko HJ, Huh YM, Kim HT, Kim SH. 2008. Implantation of human umbilical cordderived mesenchymal stem cells as a neuroprotective therapy for ischemic stroke in rats. Brain Res. 1229:233–248. doi:10.1016/j.brainres.2008.06.087.
Lazarov O, Mattson MP, Peterson DA, Pimplikar SW, van Praag H. 2010. When neurogenesis encounters aging and disease. Trends Neurosci. 33(12):569–579. doi:10.1016/j.tins.2010.09.003.
Lee HH, Shin MS, Kim YS, Yang HY, Chang HK, Lee TH, Kim CJ, Cho S, Hong SP. 2005. Early treadmill exercise decreases intrastriatal hemorrhageinduced neuronal cell death and increases cell proliferation in the dentate gyrus of streptozotocininduced hyperglycemic rats. J Diabetes Complications. 19(6):339– 346. doi:10.1016/j.jdiacomp.2005.03.006.
li Ming G, Song H. 2011. Adult Neurogenesis in the Mammalian Brain: Significant Answers and Significant Questions. Neuron. 70(4):687–702. doi:10.1016/j.neuron.2011.05.001.
Lin R, Cai J, Nathan C, Wei X, Schleidt S, Rosenwasser R, Iacovitti L. 2015. Neurogenesis is enhanced by stroke in multiple new stem cell niches along the ventricular system at sites of high BBB permeability. Neurobiol Dis. 74:229–239. doi:10.1016/j.nbd.2014.11.016.
Medicetty S, Bledsoe AR, Fahrenholtz CB, Troyer D, Weiss ML. 2004. Transplantation of pig stem cells into rat brain: Proliferation during the first 8 weeks. Exp Neurol. 190(1):32–41. doi:10.1016/j.expneurol.2004.06.023.
Meltzer LA, Yabaluri R, Deisseroth K. 2005. A role for circuit homeostasis in adult neurogenesis. Trends Neurosci. 28(12):653–660. doi:10.1016/j.tins.2005.09.007.
Mitchell KE, Weiss ML, Mitchell BM, Martin P, Davis D, Morales L, Helwig B, Beerenstrauch M, AbouEasa K, Hildreth T, Troyer D. 2003. Matrix Cells from Wharton’s Jelly Form Neurons and Glia. Stem Cells. 21(1):50–60. doi:10.1634/stemcells.21150.
Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, Zhao Z, Toga AW, Jacobs RE, Liu CY, Amezcua L, Harrington MG, Chui HC, Law M, Zlokovic BV. 2015. BloodBrain barrier breakdown in the aging human hippocampus. Neuron. 85(2):296–302. doi:10.1016/j.neuron.2014.12.032.
Nekanti U, Rao VB, Bahirvani AG, Jan M, Totey S, Ta M. 2010. Longterm expansion and pluripotent marker array analysis of Wharton’s jellyderived mesenchymal stem cells. Stem Cells and Development 19(1):117–130. doi:10.1089/scd.2009.0177.
Pera MF, Reubinoff B, Trounson A. 2000. Human embryonic stem cells. J Cell Sci. 113(1):5–10. doi:10.1242/jcs.113.1.5.
Ramírez BG, Blázquez C, Gómez Del Pulgar T, Guzmán M, De Ceballos ML. 2005. Prevention of Alzheimer’s disease pathology by cannabinoids: Neuroprotection mediated by blockade of microglial activation. J Neurosci. 25(8):1904–1913. doi:10.1523/JNEUROSCI.454004.2005.
Sierra A, Encinas JM, Deudero JJ, Chancey JH, Enikolopov G, OverstreetWadiche LS, Tsirka SE, MaleticSavatic M. 2010. Microglia shape adult hippocampal neurogenesis through apoptosiscoupled phagocytosis. Cell Stem Cell. 7(4):483–495. doi:10.1016/j.stem.2010.08.014.
Smith AD. 1980. Age differences in encoding, storage, and retrieval. In: LW Poon, JL Fozard, LS Cermak, D Arenberg, LW Thompson, editors, New Directions in Memory and Aging: Proceedings of the George A. Talland Memorial Conference. Hove. p. 24–45. doi:10.4324/9781315774886.
Solaroglu I, Cahill J, Tsubokawa T, Beskonakli E, Zhang JH. 2009. Granulocyte colonystimulating factor protects the brain against experimental stroke via inhibition of apoptosis and inflammation. Neurol Res. 31(2):167–172. doi:10.1179/174313209X393582.
Speisman RB, Kumar A, Rani A, Foster TC, Ormerod BK. 2013. Daily exercise improves memory, stimulates hippocampal neurogenesis and modulates immune and neuroimmune cytokines in aging rats. Brain, Behav, Immun. 28:25–43. doi:10.1016/j.bbi.2012.09.013. Sun Y, Jin K, Xie L, Childs J, Mao XO, Logvinova A, Greenberg DA. 2003.
VEGFinduced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest. 111(12):1843–1851. doi:10.1172/JCI200317977.
Terry AV, Kutiyanawalla A, Pillai A. 2011. Agedependent alterations in nerve growth factor (NGF) related proteins, sortilin, and learning and memory in rats. Physiol Behav. 102(2):149–157. doi:10.1016/j.physbeh.2010.11.005.
Villeda SA, Plambeck KE, Middeldorp J, Castellano JM, Mosher KI, Luo J, Smith LK, Bieri G, Lin K, Berdnik D, et al. 2014. Young blood reverses agerelated impairments in cognitive function and synaptic plasticity in mice. Nat Med. 20(6):659–663. doi:10.1038/nm.3569.
Wang HS, Hung SC, Peng ST, Huang CC, Wei HM, Guo YJ, Fu YS, Lai MC, Chen CC. 2004. Mesenchymal Stem Cells in the Wharton’s Jelly of the Human Umbilical Cord. Stem Cells. 22(7):1330–1337. doi:10.1634/stemcells.20040013.
Weiss ML, Anderson C, Medicetty S, Seshareddy KB, Weiss RJ, VanderWerff I, Troyer D, McIntosh KR. 2008. Immune Properties of Human Umbilical Cord Wharton’s JellyDerived Cells. Stem Cells. 26(11):2865–2874. doi:10.1634/stemcells.2007 1028.
Weiss ML, Medicetty S, Bledsoe AR, Rachakatla RS, Choi M, Merchav S, Luo Y, Rao MS, Velagaleti G, Troyer D. 2006. Human Umbilical Cord Matrix Stem Cells: Preliminary Characterization and Effect of Transplantation in a Rodent Model of Parkinson’s Disease. Stem Cells. 24(3):781–792. doi:10.1634/stemcells.20050330.
Weiss ML, Mitchell KE, Hix JE, Medicetty S, ElZarkouny SZ, Grieger D, Troyer DL. 2003. Transplantation of porcine umbilical cord matrix cells into the rat brain. Exp Neurol. 182(2):288–299. doi:10.1016/S00144886(03)001286.
Yang CC, Shih YH, Ko MH, Hsu SY, Cheng H, Fu YS. 2008. Transplantation of human umbilical mesenchymal stem cells from Wharton’s jelly after complete transection of the rat spinal cord. PLoS ONE. 3(10). doi:10.1371/journal.pone.0003336.
Yata K, Matchett GA, Tsubokawa T, Tang J, Kanamaru K, Zhang JH. 2007. Granulocytecolony stimulating factor inhibits apoptotic neuron loss after neonatal hypoxiaischemia in rats. Brain Res. 1145(1):227– 238. doi:10.1016/j.brainres.2007.01.144.
Zhou C, Yang B, Tian Y, Jiao H, Zheng W, Wang J, Guan F. 2011. Immunomodulatory effect of human umbilical cord Wharton’s jellyderived mesenchymal stem cells on lymphocytes. Cell Immunol. 272(1):33–38. doi:10.1016/j.cellimm.2011.09.010.
Article Metrics
Refbacks
- There are currently no refbacks.
Copyright (c) 2021 The Author(s)

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.









