HYDROTALSIT Zn-Al-EDTA SEBAGAI ADSORBEN UNTUK POLUTAN ION Pb(II) DI LINGKUNGAN Zn-Al-EDTA Hydrotalcite as Adsorbent for Pb(II) Ion Pollutant in The Environment)
Roto Roto(1*), Dahlia Rosma Indah(2), Agus Kuncaka(3)
(1) Jurusan Kimia, Fakultas Matematika dan Ilmu Pengetahuan Alam, Universitas Gadjah Mada, Sekip Utara Yogyakarta 55281 Indonesia
(2) Jurusan Kimia, Fakultas Matematika dan Ilmu Pengetahuan Alam, Universitas Gadjah Mada, Sekip Utara Yogyakarta 55281 Indonesia
(3) Jurusan Kimia, Fakultas Matematika dan Ilmu Pengetahuan Alam, Universitas Gadjah Mada, Sekip Utara Yogyakarta 55281 Indonesia
(*) Corresponding Author
Abstract
ABSTRAK
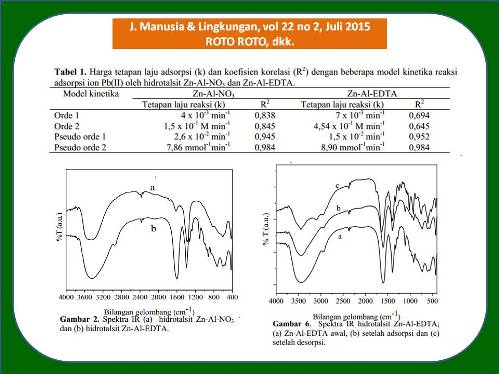
Polusi ion Pb(II) di dalam lingkungan perairan cenderung naik seiring peningkatan jumlah industri smelter dan daur ulang aki bekas. Penelitian ini bertujuan untuk menguji kemampuan hidrotalsit Zn-Al-EDTA sebagai adsorben ion Pb(II) dalam air secara mendalam. Hidrotalsit Zn-Al-NO3 disintesis dengan metode kopresipitasi dan hidrotermal pada temperatur 100 °C selama 15 jam. Hidrotalsit Zn-Al-EDTA diperoleh dengan penukaran ion. Keasaman larutan, kinetika dan kapasitas adsorpsi diteliti. Hidrotalsit Zn-Al-EDTA memiliki d003 sebesar 14,52 Å sementara Zn-Al-NO3 sebesar 8,90 Å. Spektra FTIR menunjukkan keberadaan serapan gugus C=O pada bilangan gelombang 1684,77 cm-1. Kondisi optimum adsorpsi ion Pb(II) terjadi pada pH 4, waktu kontak 60 menit dan kapasitas adsorpsi diperoleh 2,07 mg/g pada konsentrasi awal 10 mg/L dengan berat adsorben 0,100 g. Adsorpsi ion Pb(II) oleh hidrotalsit Zn-Al-EDTA mengikuti reaksi pseudo orde dua dengan tetapan laju adsorpsi sebesar 8,90 g mmol-1min-1. Adsorpsi ion Pb(II) oleh Zn-Al-EDTA terjadi karena pembentukan khelat Pb-EDTA di dalam struktur hidrotalsit. Hasil ini diharapkan mampu memberikan kontribusi yang lebih luas di dalam pengendalian konsentrasi Pb(II) di lingkungan.
ABSTRACT
Polution by Pb(II) ion in the water environment tends to increase due the increase in the number of lead smelter and lead acid battery recycling industries. This work aims at studying in details the ability of Zn-Al-EDTA hydrotalcite as adsorbent for Pb(II) ion in the environment. The Zn-Al-NO3 hydrotalcite was synthesized first by coprecipitation method followed by hydrothermal treatment at 100 °C for 15 h. The Zn-Al-EDTA hydrotalcite was later obtained by ion exchange process. The solution pH, kinetics and adsorption capacity were studied. The XRD data showed that Zn-Al-EDTA and Zn-Al-NO3 hydrotalcites have d003 of 14.52 and 8.90 Å, respectively. The FTIR spectra suggested that C=O group was observed with absorption band at 1684.77 cm-1. The optimum condition for adsorption of Pb(II) ion by Zn-Al-EDTA hydrotalcite was obtained at pH 4, contact time of 60 minutes and adsorption capacity of 2.07 mg/g at initial concentration of 10 mg/L for each 0.100 g of adsorbent. The Pb(II) ion adsorption by Zn-Al-EDTA follows pseudo second order of reaction with reaction rate constant of 8,90 g mmol-1min-1. The increase in adsorption of Pb(II) ion by hydrotalcite Zn-Al-EDTA is believed to be due to the formation of chelate complex between Pb(II) and EDTA in the interlayer space of hydrotalcite Zn-Al-EDTA. This finding is expected to find broad applications for controlling Pb(II) in the environment.
Keywords
Full Text:
Artikel lengkap (PDF) (Bahasa Indonesia) Gambar 1 (Bahasa Indonesia) Gambar 2 (Bahasa Indonesia) Gambar 3 (Bahasa Indonesia) Gambar 4 (Bahasa Indonesia) Gambar 5 (Bahasa Indonesia) Gambar 6 (Bahasa Indonesia)References
Cavani, F., Trifirò, F., dan Vaccari, A., 1991. Hydrotalcite-Type Anionic Clays: Preparation, Properties and Applications. Catal. Today, 11(2):173-301.
Doglin, Z., Sheng, G., Hu, J., Chen, C., dan Wang, X., 2011. The Adsorption of Pb(II) on Mg2Al Layered Double Hydroxide. Chem. Eng. J., 171:167-174.
Park, M., Choi, C.L.,. Seo, Y.J, Yeo, S.K., Choi J., Komarneni, S. dan Lee, J.H., 2007. Reactions of Cu2+ and Pb2+ with Mg/Al Layered Double Hydroxide. Applied Clay Sci., 37(1–2):143–148.
Pérez, M.R., Pavlovic, I., Barriga, C., Cornejo, J., Hermosín, M.C., dan Ulibarri, M.A., 2006. Uptake of Cu2+, Cd2+ and Pb2+ on Zn-Al Layered Double Hydroxide Intercalated with EDTA. Applied Clay Sci., 32:245-251.
Rives, V., 2001. Study of Layered Double Hydroxides by Thermal Methods. In Layered Double Hydroxides: Present and Future. Rives, V., Ed., Nova Science Publishers Inc., New York.
Roto, R., 2005. Electron and Ion Transport in Redox Active Transition Metal Layer Double Hydroxide. Ph.D. Thesis, University of New Brunswick, New Brunswick.
Seida, Y., Nakano, Y. dan Nakamura, Y., 2001. Rapid Removal of Dilute Lead from Water by Pyroaurite-Like Compound, Water Research, 35:2341–2346.
Stumm, W. dan Morgan, J.J., 1996. Aquatic Chemistry, Chemical Equilibria and Rates in Natural Waters, 3rd ed., John Wiley & Sons, Inc., New York, 1022.
Ureña-Amate, M.D., Boutarbouch, N.D., Socias-Viciana, M.M. dan González-Pradas, E., 2011. Controlled Release of Nitrate from Hydrotalcite Modified Formulations, Applied Clay Science, 52(4):368-373.
Violante, A., Pucci, M., Cozzolino, V., Zhu, J. dan Pigna, M., 2009. Sorption/Desorption of Arsenate on/from Mg–Al Layered Double Hydroxides: Influence of Phosphate, J. Colloid Interface Sci., 333(1):63-70.
Widowati, W., Sastiono, A., dan Jusuf, R., 2008. Efek Toksik Logam-Pencegahan dan Penanggulangan Pencemaran. Andi Publisher, Yogyakarta.
Xiao, L., Ma, W., Han, M. dan Cheng, Z., 2011. The Influence of Ferric Iron in Calcined Nano-Mg/Al Hydrotalcite on Adsorption of Cr (VI) from Aqueous Solution, Journal of Hazardous Materials, 186(1):690-698.
Xie, X., An, X., Wang, X. dan Wang, Z., 2003. Preparation, Characterization and Application of ZnAlLa-Hydrotalcite-Like Compounds, Journal of Natural Gas Chemistry, 12:259-263.
Article Metrics
Refbacks
- There are currently no refbacks.
Copyright (c) 2017 Jurnal Manusia dan Lingkungan







