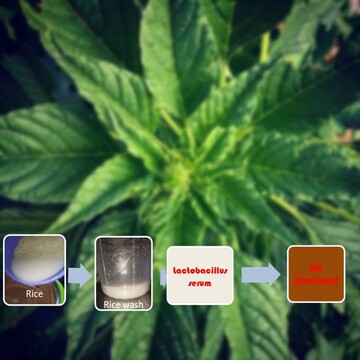
Influence of ‘Lactobacillus serum’ on the growth of Amaranthus hybridus and some soil chemical properties under screen house conditions
Ikponmwosa Ogbemudia(1*), Valerie Ifeyinwa Ofili Edosa(2), Enameguono Ekpemuaka(3)
(1) Department of Soil Science and Land Management, Faculty of Agriculture, University of Benin, Benin City, Nigeria
(2) Department of Soil Science and Land Management, Faculty of Agriculture, University of Benin, Benin City, Nigeria
(3) Department of Soil Science and Land Management, Faculty of Agriculture, University of Benin, Benin City, Nigeria
(*) Corresponding Author
Abstract
The potentials of lactic acid bacteria serum, termed as ‘Lactobacillus (LB) serum’, in enhancing soil nutrient availability and supplies for the growth of Amaranthus hybridus and some chemical properties of soil were investigated at the screen house of the Department of Soil Science, University of Benin. Three application rates of the serum were adopted, consisting of 3 mL (3,000 L.ha-1), 5 mL (5,000 L.ha-1), and 0 ml (0 L.ha-1), and represented as treatment. Amaranthus hybridus was transplanted into pots containing 2 kg of 2 mm sieved air-dried soil. Each treatment was replicated seven times to give a total of 21 pots that were laid out in a Completely Randomized Design (CRD). The treatments were applied twice a week starting from the 2nd week after transplanting. The plant growth indices measured were number of leaves, plant height and plant biomass. The results showed that serum positively influenced the number of leaves and plant biomass (4.333 kg to 4.830 kg) compared to the control (3.901 kg). However, the highest value of the plant biomass was found in the 3 mL (3,000 L.ha-1) treated pots, while the microbial colonies of bacteria in soil after serum application were sustained when compared with the control but at a reduced population for Bacillus subtilis. The application of LB. serum slightly improved the soil total organic carbon (320.0 g.kg-1 to 352.0 g.kg-1) and nitrogen (3.102 g.kg-1 to 3.325 g.kg-1) as against, 64.00 g.kg-1, and 0.639 g.kg-1 in the control respectively.
Keywords
Full Text:
PDFReferences
Agbemin, J. O. (2020). The environmental chemistry of soils and sediments. Ibadan, Nigeria: University of Ibadan Press. pp. 603–635.
Alejandro, J.G., Lorena, C.L., Maria, F.B and Raul, R. (2017). Plant probotic bacteria enhance the quality of fruits and horticultural crops. AIMS Microbiology, 13(3), pp. 483-501.
Bray, R.H. and Kurtz, L.T. (1945) Determination of total, organic and available forms of phosphorus in soils. Soil Science, 59(1), pp. 39-45.
Bremner, J. M. and Mulvaney C. C. (1982). Total nitrogen determination. In: Page, A.L. et al. eds., Methods of Soil Analysis. Part 2 Chemical and Microbiological Properties, 2nd ed. Agronomy Monograph No. 9, Madison, Wisconsin: American Society of Agronomy, Soil Science Society of America, pp. 199 – 224.
Bridson, E. Y. (2006). The oxoid manual, 9th ed. Basingstoke Hamsphire, England: Oxoid Limited., pp. 623.
Burt, R. (2014). Soil survey field and laboratory methods. manual soil survey investigation reports, Vol. 51. Washington, DC: Department of Agriculture, pp. 227 – 234.
Fonge, B.A., Bechem, E.E and Awo, E.M. (2016) Fertilizer rate on the growth, yield and nutrient concentration of leafy vegetables. International Journal of Vegetable Science, 22(3), pp. 274 - 288.
Islam, M. T. and Hussain, M. M. (2012). Plant Probiotics in Phosphorus Nutrition in Crops, with Special Reference to Rice. In: Maheshwari, D.K, ed., Bacteria in Agrobiology: Plant Probiotics. Berlin- Heidelberg: Springer, pp. 325–363.
Kang, A. (2019). Lactobacillus in soil remediation. ACTA Scientific Microbiology, 2(6), pp. 71 – 72.
Kwenin, W.K.J., Wolli, M., and Dzomeku, B.M. (2011). Assessing the nutritional value of some African indigenous green leafy vegetables in Ghana. Journal of Animal Plant Science, 10(13), pp. 1300 – 1305.
Maclean, E.O. and Carbonell, M.D. (1972). Calcium magnesium and potassium saturation ratio in two soils and their effects on yields and nutrient contents of German millet and alfafa. Soil Science Society of America, 36(6), pp. 927-930.
McGrath, B. (2011). How to Capture, Cultivate, Preserve and Use Lactic Acid Bacteria (Pro-Kashi Korean Natural Farming).
Mohammed, M.I., and Sharif, N. (2011). Mineral composition of some leafy vegetables consumed in Kano State, Nigeria. Nigerian Journal of Basic Applied Science, 19(2), pp. 208-211.
Nelson, D.W. and Sommers, L.E. (1982). Total carbon and organic matter In: Page, A.L. et al., eds., Methods Of Soil Analysis Part 2 Agron. Monogra – 9. 2nd ed. Madison Wisc: ASA and SSSA., pp. 539 – 579.
Ogbemudia, I., Elenwo, C.E, and Oghenerugba, F. (2019). Growth of amaranth (Celosia Spp.) and soil microbial changes as influenced by rubber effluent and poultry manure in an ultisol in the lowland rain forest zone of Nigeria. International Journal of Research and Innovation in Applied Science, 4, pp. 2454-6194.
Ogunlade, M.O., Adeyemi, E.A., Ogunleti, D.O., and Ibiyomi, P. S. (2011). Effect of cocoa pod husk, urea fortified cocoa pod husk and NPK fertilizers on the growth and yield of Solanum macrocarpon cultivation. International Journal of Organic Agriculture Research and Development, 3, pp. 1–8.
Okhuoya, J.A., Okungbowa, F.I., and Shittu, H.O. (2012). Biological techniques and applications. a comprehensive teaching manual. Benin: Uniben Press. pp. 393-407.
Olowoake, A. A. and Ojo, J. A. (2014). Effect of fertilizer types on the growth and yield of Amaranthus caudatus in Ilorin, Southern Guinea, Savanna Zone of Nigeria. Nigeria: Department of Crop Production, Kwara State University.
Rashid, M., Khalil, S., Ayub, N., Alam, S., and Latif, F. (2004). Organic acid production and phosphate solubilization by phosphate solubilizing microorganisms (PSM) under in-vitro conditions. Pakistan Journal of Biological Sciences, 7(2), pp. 187 – 196.
Shiyam, J. O. and Binang, W. B. (2011). Effect of poultry manure and urea- n on flowering occurrence and leaf productivity of Amaranthus cruentus, Journal of Applied Sciences and Environmental Management, 15(1), pp. 13– 15.
Singh, P., Kumar, V. and Agrawal, S. (2014) Evauation of phytase producing bacteria for their plant growth promoting activities. International Journal of Microbiology, pp. 1-8.
Thomas, G.W. (1982). Exchangeable Cations. In: A.L. Page et al., ed., . Methods Of Soil Analysis Part 2, 2nd ed. Madison, WI. P : ASA and SSSA, pp. 159-165.
Wernli, M. (2014). Growing Lactobacilli inocula: self-sufficient fermentation of organic matter in Terra Preta sanitation. Canberra: Canberra University, pp. 1 – 10.
Willey, L. M., Sherwood, L. J., and Woolverton, L. (2008). Microbiology. 7th ed. New York: Mc-Graw Hill, pp. 966.
Article Metrics
Refbacks
- There are currently no refbacks.
Ilmu Pertanian (Agricultural Science) ISSN 0126-4214 (print), ISSN 2527-7162 (online) is published by Faculty of Agriculture Universitas Gadjah Mada collaboration with Perhimpunan Sarjana Pertanian Indonesia (PISPI) and licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.












_2025_-_kecil_.png)
_2024_kecil_2.png)
